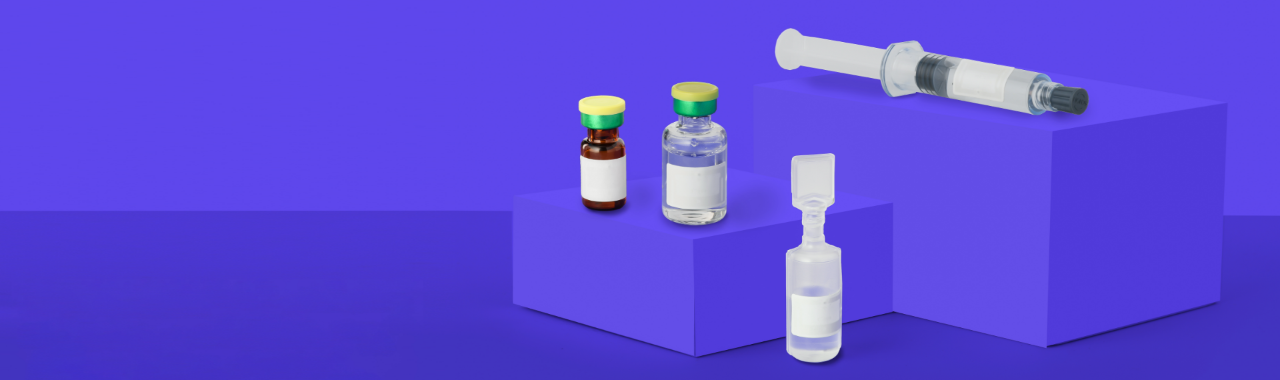
GLENDALE, CA – February 10, 2022 — Avery Dennison Smartrac today launched its AD Minidose U9 RAIN RFID inlay for pharmaceutical applications, unlocking critical RFID value for healthcare, pharmacies, and laboratory asset management. AD Minidose U9 is one of the smallest products on the market to receive ARC certification (Spec S) from Auburn University’s RFID Lab, and to be approved for use by the DoseID industry consortium.
AD Minidose U9 builds on the success of the Minidose U8 inlay launched in 2021, and performs in the standard UHF RFID frequency band between 860MHz-960MHz, with a small form factor of 22x12mm. With its long read range, even in densely packed inventory environments, AD Minidose U9 is certified to work on all current DoseID product categories. The inlay uses NXP’s proven UCODE®️ 9 IC, which is equipped with 96 bits of EPC memory, including a 96-bit Tag IDentifier (TID) with a 48-bit unique serial number factory-encoded into the TID.
Max Winograd, vice president, connected products at Avery Dennison Smartrac, said, “AD Minidose U9 meets the needs for a wide range of pharmaceutical item-level use cases. The inlay performs robustly on clear and amber glass as well as plastics and syringes, even when filled with pharmaceuticals and biologicals. This unlocks critical RFID value for asset and inventory management in multiple pharma or healthcare applications. It also meets specific customer requirements with the goal of ensuring patient safety, increasing nursing “time to care,” and decreasing inefficiencies in the operational process.”
Avery Dennison Smartrac is a key RFID partner for Kit Check®, a leading provider of automated medication tracking and diversion detection solutions for hospital pharmacies in the U.S. and has supplied RFID tags for the Kit Check application since its inception. The Kit Check RFID-enabled inventory management solution helps hospitals modernize restocking processes and automates them to save redundant drug spend and ensure patient safety.
According to Tim Kress-Spatz, Kit Check co-founder, “Kit Check's inventory of pharmaceutical containers continues to expand by adding different series of containers in various sizes. We pursue to work successfully with Avery Dennison Smartrac, and their product line continuously evolves to meet our needs. We tag a myriad of pharmaceuticals through the Kit Check solution to deliver the right medicine, to the right patient at the right time, every time."
Available as wet and dry inlays with the NXP UCODE 9 IC, AD Minidose U9 will ship in volume from Q2 2022. AD Minidose U9 inlays are compliant with ISO 9001:2015 Quality Management and ISO 14001:2015 Environmental Management, which ensures a reliable and state-of-the-art product that meets a variety of application needs. They are manufactured according to the industry’s highest quality standards, as confirmed by the RFID Lab at Auburn University, which awarded Avery Dennison Smartrac its first-ever ARC accreditation for overall design and manufacturing quality.
Media contact
Christian Achenbach
Communications Manager, Avery Dennison Smartrac
christian.achenbach@eu.averydennison.com